Research Roundup: First African approval of CAB-LA, Cancer drug with malaria potential, Ebola drug deliveries
In this regular feature on Breakthroughs, we highlight some of the most interesting reads in global health research from the past week.
Sign up to receive news and updates from GHTC.
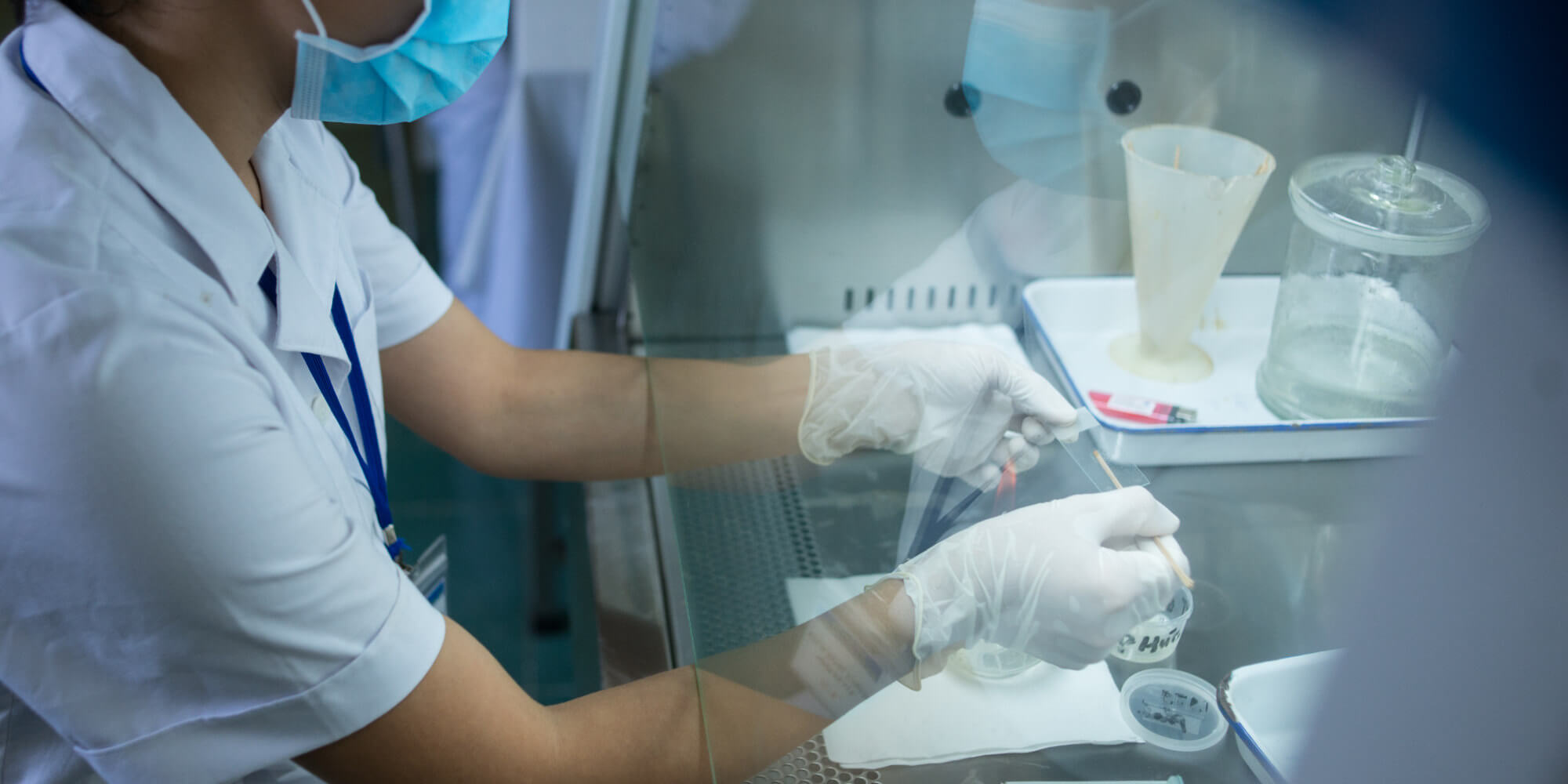
In this regular feature on Breakthroughs, we highlight some of the most interesting reads in global health research from the past week.
In observance of World Polio Day on October 24, GHTC is reflecting on the biggest news about this year’s polio outbreaks and on the connection between this year’s outbreaks and polio vaccines, as well as the potential of new vaccines to contribute to eradication.
In this regular feature on Breakthroughs, we highlight some of the most interesting reads in global health research from the past week.
The 2022 World Health Summit, taking place from October 16 to 18, brings together scientific, political, private, and civil society sectors to discuss the achievement of health for all. It is an opportunity to draw attention to the fact that pregnant women are underserved by currently available antimalarial therapies. Medicines for Malaria Venture (MMV), through its Malaria in mothers and babies (MiMBa) strategy, is working with partners to close critical gaps in the availability of well-tolerated malaria prevention and treatment methods for pregnant women to achieve true health for all.