Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
The US Food and Drug Administration (FDA) approved the first 3D-printed drug last week, paving the way for the technology to be used to customize medical care to individual patients. 3D printing is a manufacturing process that involves using a computer to create an object one layer at a time. In the case of the recently approved Spritam—a treatment for epilepsy—the printing allowed for greater control over formulation of the drugs, resulting in the creation of the first 1,000 milligram tablets that remain easily dissolvable despite their large size. The use of 3D printers could ultimately enable hospitals to produce their own medicines, adjusting the dose for individual patients. Meanwhile, at the University College London School of Pharmacy, researchers are exploring the possibility of using 3D printing to create uniquely shaped drugs. Drugs are absorbed at different rates depending on the surface area and volume of the tablet, and by adjusting the shape of a tablet (i.e., creating a pyramid or doughnut shaped pill) the rate at which a drug’s active ingredients are released could be manipulated.
Leaders at the FDA and the US Department of Health and Human Services propose mechanisms and protocol to expedite drug development in public health emergencies in The New England Journal of Medicine, stating “if we are to act on lessons learned [from the Ebola outbreak] there is no time to waste.” The authors note that limited research capacity delayed the testing of experimental treatments, calling for efforts to strengthen clinical trial infrastructure globally. The outbreak also prompted debate about research ethics in emergency settings (i.e., whether it was ethical to administer untested therapies and the ethics of using placebos in an outbreak). Consequently, the authors recommend that processes for ethical review of studies in outbreaks be streamlined. Finally, the authors stress the need for coordination between health and regulatory authorities, researchers, and product developers. In the Ebola outbreak, the US government partnered with West African governments, academic institutions in both regions, and product developers at every stage. Clinical trials of different therapies were conducted concurrently, allowing partners to have a single, shared control group. The authors note that to facilitate collaboration for rapid drug development in outbreaks, there is a need to establish “model agreements” regarding data management and intellectual property rights.
BiondVax Pharmaceuticals announced last week that those vaccinated with an experimental flu vaccine in 2012 had stronger immune responses to strands of influenza that didn’t even exist three years ago. The vaccine was developed to provide broad protection against different strands of influenza, in contrast to the seasonal flu vaccine which protects against the three to four strands of influenza predicted to be the most virulent that year. In 2012, the vaccine (M-001) was tested in phase II clinical trials in which 90 participants—all aged 65 or older—received the vaccine and 30 served as the control group. The vaccine successfully triggered an immune response, including the development of antibodies against several different strains of influenza. Earlier this year, BiondVax exposed blood plasma collected from participants in the 2012 trial to a strain of influenza that did not exist in 2012, and discovered protective antibodies against the strain in 50 percent of the plasma samples from those that received the vaccine, compared to just 10 percent of the samples from those in the control group. As BiondVax’s President Ron Babecoff explains, the vaccine “provides a safety net by broadening the immunogenicity [immune response] to existing and future flu strains.” Next, the vaccine will be tested in phase 3 clinical trials.
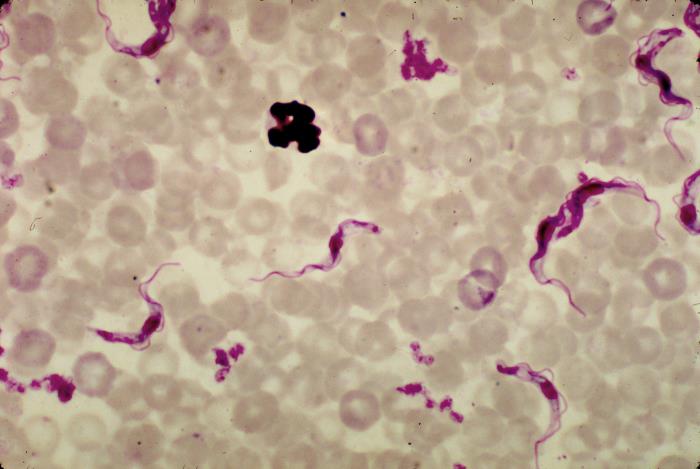
A simple innovation—blue cloth and fine netting sprayed with insecticide between two sticks—could wipe out African sleeping sickness in a mere five to six years. African sleeping sickness is transmitted by tsetse flies, which breed along rivers looking for “something that contrasts with the green vegetation,” according to entomologist Steve Torr, “and for some reason they’re especially attracted to bright blue.” The flies are attracted to the blue cloth and the insecticide kills them within three minutes. The contraption is currently being tested in northern Uganda, where more than 17,000 have been installed over 1,500 square miles; in less than two years, the population of tsetse flies has been reduced by 90 percent. Only 1 in 1,000 flies carry the parasite, and the flies only reproduce once every nine days—whereas mosquitoes lay hundreds of eggs each day—bringing Torr’s team to the estimate of five to six years to both reduce the tsetse population, and eliminate the parasite from the remaining population.