Led US COVID-19 product development efforts for vaccines, diagnostics, and therapeutics, supporting dozens of innovations, including all vaccines approved or authorized by the US Food and Drug Administration (FDA).
Global Health R&D across the US government | BARDA
Global Health R&D at the Biomedical Advanced Research and Development Authority
 What does BARDA do for global health R&D?
What does BARDA do for global health R&D?
The Biomedical Advanced Research and Development Authority (BARDA) supports the development of vaccines, drugs, and other medical countermeasures (MCMs) to protect Americans against threats to public health, including emerging infectious diseases, pandemic influenza, and antimicrobial resistance (AMR).
 Why is BARDA’s role in global health R&D important?
Why is BARDA’s role in global health R&D important?
BARDA works with industry and other partners to bridge the “valley of death” between basic research and product development, where research and development (R&D) efforts most often fail. Through unique contracting and incentive mechanisms, BARDA’s partnerships ensure promising research is translated into urgently needed medical products by creating commercial incentives for developers that would otherwise not exist. During the COVID-19 pandemic, BARDA’s prominence and funding has grown exponentially as the agency was charged with leading the US government’s MCM R&D portfolio, demonstrating how—with sufficient, sustained funding—BARDA is uniquely equipped to advance products against a range of other global health threats.
 BARDA support has helped advance:
BARDA support has helped advance:
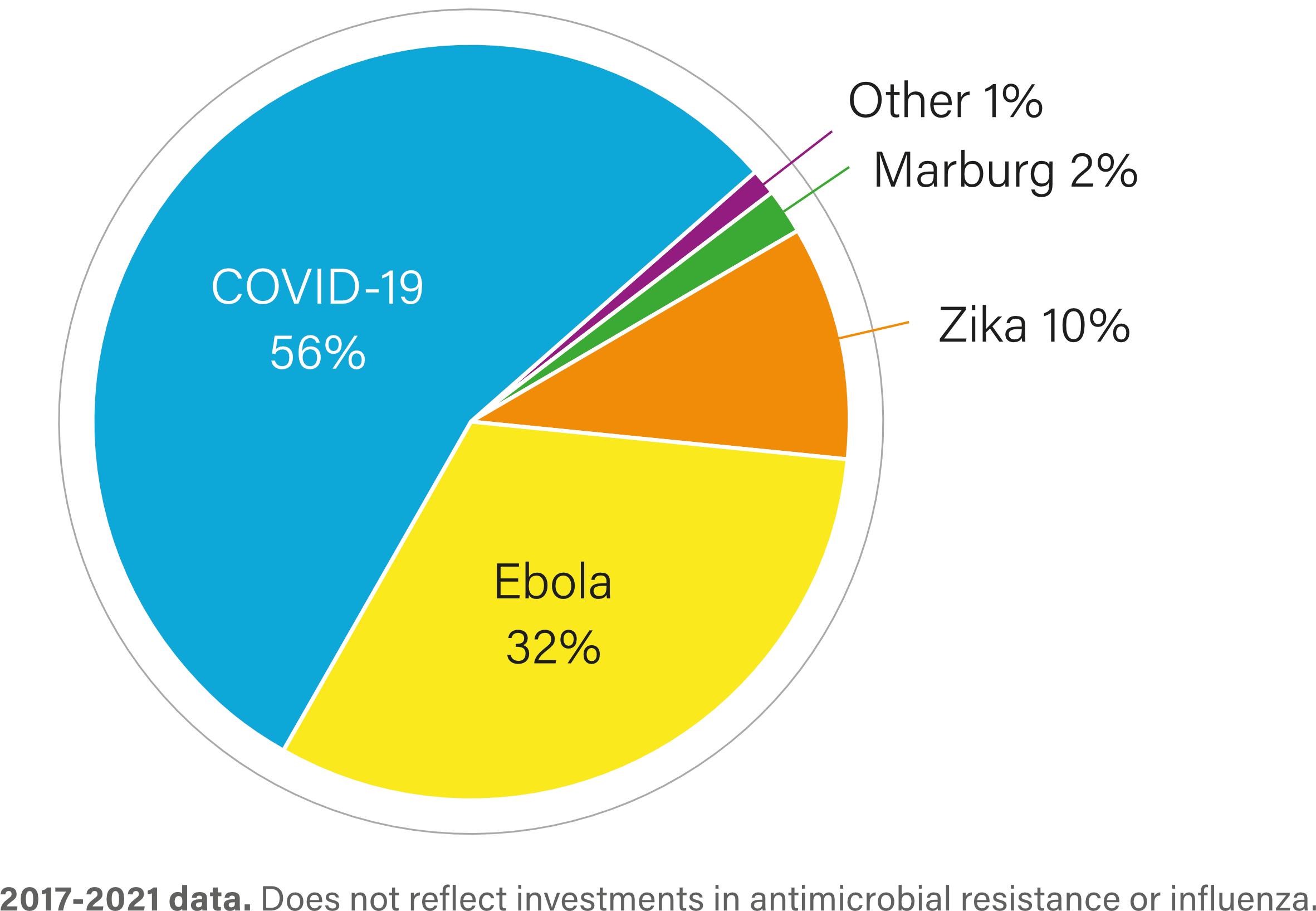
 R&D investment by health area
R&D investment by health area
IMPACT OF INVESTMENT
COVID-19
EBOLA
Development of the world’s first Ebola vaccine, as well as two Ebola treatments and one rapid diagnostic test approved by the FDA.
AMR
Accelerating antibacterial research through the CARB-X public-private partnership, which has supported 92 projects in 12 countries, to build the world’s largest early development pipeline of antibacterial innovations.
MPOX
Development of the only FDA-approved vaccine for smallpox/Mpox, which was deployed during the 2022–2023 outbreak.
ZIKA
Development of six FDA-approved diagnostics, including tests to identify infection and screen blood supplies.
INFLUENZA
Strengthening manufacturing capacity in low- and middle-income countries to enable rapid production of seasonal and pandemic influenza vaccines.
