In this guest post, Dr. Jayasree K. Iyer—Chief Scientific Officer at the Access to Medicine Foundation—writes about the findings from their newest study, which examines the current state of the vaccine pipeline and how large pharmaceutical companies are considering access to these products.
 Vaccine manufacturers play a critical role in innovating new vaccines and making existing vaccines more suitable for use in developing countries.
Apart from developing effective, appropriate vaccines, manufacturers work with relevant stakeholders to ensure that access to these vaccines is
ramped up upon market introduction, addressing issues like supply and affordability. Vaccines are the cornerstone of disease prevention: mass immunization
has led to the eradication of smallpox, and between 2000 and 2010, it helped cut the global childhood mortality rate in half. Yet many people cannot
access full immunization, especially with newer vaccines. Recent attempts to boost immunization coverage (e.g., of the relatively new pneumococcal
and rotavirus vaccines) shows that immunization coverage and access can be increased when good vaccines, committed funding, and strong global partnerships
come together.
Vaccine manufacturers play a critical role in innovating new vaccines and making existing vaccines more suitable for use in developing countries.
Apart from developing effective, appropriate vaccines, manufacturers work with relevant stakeholders to ensure that access to these vaccines is
ramped up upon market introduction, addressing issues like supply and affordability. Vaccines are the cornerstone of disease prevention: mass immunization
has led to the eradication of smallpox, and between 2000 and 2010, it helped cut the global childhood mortality rate in half. Yet many people cannot
access full immunization, especially with newer vaccines. Recent attempts to boost immunization coverage (e.g., of the relatively new pneumococcal
and rotavirus vaccines) shows that immunization coverage and access can be increased when good vaccines, committed funding, and strong global partnerships
come together.
The vaccine R&D challenge
The challenge with vaccine development and access is twofold: the need for new vaccines for diseases where there are no vaccines on
the market (e.g., for HIV and AIDS, malaria, and for almost all neglected tropical diseases (NTDs)) and the need for vaccines that are more suitable for use in the developing world (e.g., thermostable vaccines, oral, more effective vaccines and combination vaccines). The research and development
(R&D) pipeline for vaccines is primarily driven by the vaccine industry, product development partnerships (PDPs)—such as the PATH Malaria
Vaccine Initiative (MVI) and Sabin Vaccine Institute—and other research institutes. With the recent outbreak of Ebola, it is clear that vaccine
innovators play a crucial role in the development of safe, quality, effective vaccines, and that R&D must be stimulated by donors and regulators,
as well as by market forces.
Tracking vaccine development
The Access to Medicine Foundation has been tracking the development and deployment of medicines and vaccines for use in developing countries since
2008. Our almost ten years of research into the world’s 20 largest research-based pharmaceutical companies has given rise to special insight into the opportunities for addressing specific challenges. These insights can be used to inform the work of other pharmaceutical companies, as
well as governments, investors, donors, and market shapers.
Our research prompted us to embark on the first Access to Vaccines Index (due early in 2017). While talking with vaccine stakeholders and defining
appropriate, robust metrics and analysis scopes for this new access-analysis tool, we also published a preliminary report on vaccine R&D, which offers a baseline on industry efforts in this area. Data from the 2014 Access to Medicine Index was used to report on (a) the R&D pipeline for high burden vaccines and (b) the use of mechanisms that ensure rapid access and uptake of vaccines
upon market approval (termed “access provisions”).
Promise in the pipeline
The study highlights and confirms
that the vaccine R&D pipeline covers the highest burden diseases. There appears to be relatively promising candidate vaccines emerging for
diseases where no vaccine yet exists on the market (e.g., for malaria and dengue). However the pipeline for these types of vaccines remains relatively
empty. Considering the large development costs associated with vaccine development and the high attrition rate of vaccine R&D projects, there
is little promise that other new high-need vaccines products will enter the market in the coming years.
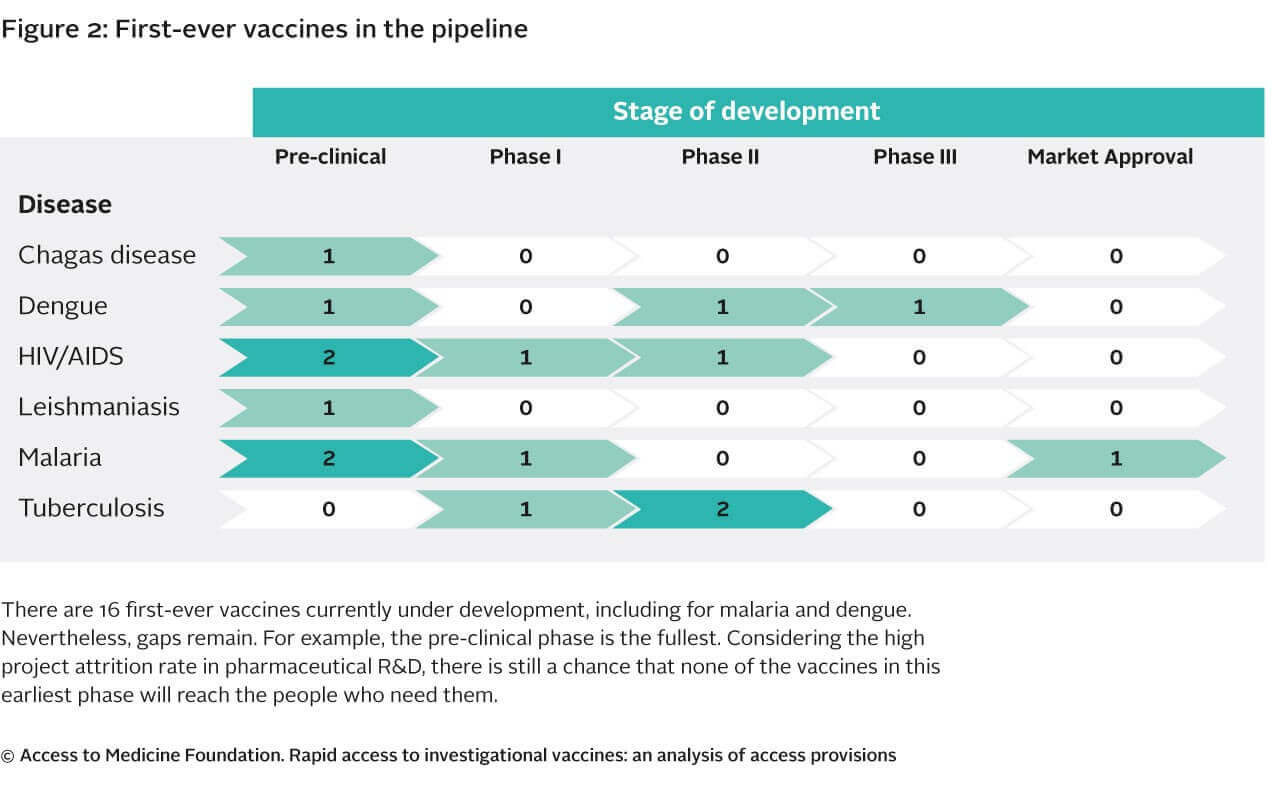
Persistent R&D gaps remain
Considering the large development costs attributed with vaccine development and the high attrition rate of projects in the pipeline, there may be few
vaccines entering the market in the coming years from industry. There may be other projects under development by research institutes, PDPs, and
biotech companies and by other smaller vaccine manufacturers. However, without significant investment (which many of the stakeholders may not have
access to), chances of successful products reaching the market remain limited. And with so many global priorities competing for investments, product
development is almost certain to take a strong hit.
Interestingly, the majority of vaccines in the pipeline are aimed at adapting or improving existing vaccines (e.g., to improve their efficacy or give
longer-lasting immunization or to develop combination vaccines), or creating the next generations of existing vaccines. Many of these projects
target leading causes of child death including diarrheal disease and pneumonia. This is important, as coverage, adherence, and acceptance is also
improved with these types of products.
Accelerating access to suitable new vaccines

- Having a vaccine on the market that meets a health need and is suitable for LMICs does not automatically resolve access issues. Photo: PATH/Aaron
Joel Santos
To drive strategic R&D decisions that meet public health needs, particularly for low- and middle-income countries, the World Health Organization
develops Preferred Product Characteristics (PPC)– recommendations for priority indications, target groups, possible immunization strategies, and desired clinical data, as well as ensuring
that vaccine presentation and packaging is suitable for low-resource settings (such as remote populations, or where infrastructure is weak). Together
these PPCs and presentation and packaging recommendations form an essential part of vaccine developers’ Target Product Profiles (TPPs). Companies
can use these TPPs to assist in defining target product characteristics, such as dosage, target price, target population, and route of administration.
Just having a vaccine in the pipeline or even on the market that meets a priority need and is suitable for use in a low- and middle-income country
will not automatically solve the world’s access problems. Companies and other stakeholders have a joint responsibility to ensure that there are
mechanisms in place to ensure the vaccine’s quick roll-out, thus ramping up coverage of vaccines upon market introduction. Companies are expected
to continuously innovate through R&D towards the goals defined in the TPP, and ensure that access provisions are aligned with the needs and
intended reach of vital products.
Big pharma actions to drive access
In our vaccine R&D study, we
found that companies have used at least one access provision in 17 percent of the vaccine projects in the pipeline. Out of 70 projects analyzed,
42 are nearing the end of the pipeline, yet only 8 projects have provisions in place for driving access once they hit the market. It is not clear
whether access provisions are truly lacking, or whether low transparency accounts for this gap. A close look at the usage of the provisions revealed
examples such as tiered pricing strategies aimed at ensuring affordability, the use of local manufacturing capacity based in a developing country
to reduce cost and ensure local supply, the use of advanced market commitments, commitments for discounted prices, and commitments to manufacture
large volumes at the point of market entry.
The systematic application of access provisions will help ensure that successful vaccine candidates are suitable for use in the countries that need
them most and align with the needs outlined in the PPCs and TPPs. This could help ensure uptake, acceptance, and coverage is ramped up upon market
entry and ultimately more lives are saved. While we indeed need clear incentives for companies to invest in vaccine pipelines, they are encouraged
to consider and put in place access provisions as early as phase II clinical stages to develop vaccine candidates with poor, rural, and isolated
populations in mind.

 Vaccine manufacturers play a critical role in innovating new vaccines and making existing vaccines more suitable for use in developing countries.
Apart from developing effective, appropriate vaccines, manufacturers work with relevant stakeholders to ensure that access to these vaccines is
ramped up upon market introduction, addressing issues like supply and affordability. Vaccines are the cornerstone of disease prevention: mass immunization
has led to the eradication of smallpox, and between 2000 and 2010, it helped cut the global childhood mortality rate in half. Yet many people cannot
access full immunization, especially with newer vaccines. Recent attempts to boost immunization coverage (e.g., of the relatively new pneumococcal
and rotavirus vaccines) shows that immunization coverage and access can be increased when good vaccines, committed funding, and strong global partnerships
come together.
Vaccine manufacturers play a critical role in innovating new vaccines and making existing vaccines more suitable for use in developing countries.
Apart from developing effective, appropriate vaccines, manufacturers work with relevant stakeholders to ensure that access to these vaccines is
ramped up upon market introduction, addressing issues like supply and affordability. Vaccines are the cornerstone of disease prevention: mass immunization
has led to the eradication of smallpox, and between 2000 and 2010, it helped cut the global childhood mortality rate in half. Yet many people cannot
access full immunization, especially with newer vaccines. Recent attempts to boost immunization coverage (e.g., of the relatively new pneumococcal
and rotavirus vaccines) shows that immunization coverage and access can be increased when good vaccines, committed funding, and strong global partnerships
come together.
