Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
A vaccine candidate against Rift Valley Fever appears to be safe and effective for livestock and has the potential for use in humans. Rift Valley Fever—which was recently identified by the World Health Organization (WHO) as a leading culprit for the next major pandemic—is often asymptomatic, or manifests with nonspecific symptoms that are easily confused with malaria. However, severe cases can involve vision impairment, brain swelling (encephalitis), hemorrhagic fever, or death. The disease is caused by a virus and transmitted to humans through mosquito bites or contact with infected animal tissue. While there is a veterinary vaccine against Rift Valley Fever used throughout Africa, it does not provide complete protection from the disease and can induce abortion. The vaccine uses a virus that causes cold-like symptoms in chimpanzees, an approach that is generally safe for humans. The candidate will next be tested in animal trials across East Africa, which will be followed by human clinical trials in the region.
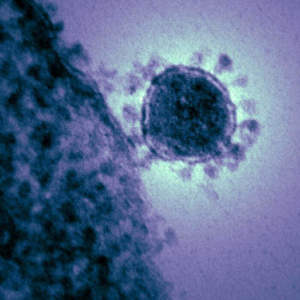
Last week, the Walter Reed Army Institute of Research (WRAIR) initiated the first-ever human clinical trials for a vaccine against the Middle East Respiratory Syndrome (MERS). The DNA-based vaccine was developed by Inovio Pharmaceuticals and GeneOne Life Sciences Inc., and the two companies are also partnering to develop vaccines against Ebola and Zika. MERS is endemic in the Middle East, however, an outbreak last spring in South Korea resulted in 186 cases and 36 deaths. The United States currently has 35,000 and 27,000 troops in the Middle East and South Korea, respectively, and as 40 percent of MERS cases are fatal, “low prevalence doesn’t mean low risk” according to Dr. Kayvon Modjarrad, associate director of the Emerging Infectious Disease Research Program at WRAIR. Meanwhile, a team of researchers at Erasmus Medical Center in Rotterdam is testing a MERS vaccine in camels in Saudi Arabia, where 12 percent of camels are infected at any time and camel-to-human transmission is a significant driver of new cases.
Inovio Pharmaceuticals and GeneOne Life Sciences Inc. are also conducting animal studies of their vaccine against Zika, and announced last week that the candidate successfully prompted an immune response in mice, including the development of antibodies against Zika. The partners will next test the candidate in primates and begin Phase 1 human clinical trials by the end of the year. However, vaccine candidates developed by Bharat Biotech in India and the US National Institutes of Health (NIH) are farthest along, according to the WHO. Bharat Biotech will launch animal studies within the next few weeks and the NIH plans to start human clinical trials of at least one of their two vaccine candidates by the end of this summer.
As more than 15 groups across government, industry, and academia have joined the hunt for a Zika vaccine, US Congressman Curt Clawson (R-FL) has introduced legislation to further incentivize Zika research and development (R&D), providing companies a 10 percent tax credit for any Zika vaccine R&D costs. The legislation was accompanied by two additional bills, the first of which would reauthorize a program—at US$200 million per year for five years—to support states with mosquito control and prevention. Clawson’s third piece of Zika-related legislation would allow supplemental funding for the Ebola response to be used for Zika, however, leaders at the NIH and US Centers for Disease Control have stated that the remaining Ebola funding has already been reprogrammed and that the agencies have had to take funding away from other programs to kickstart the Zika response.