Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
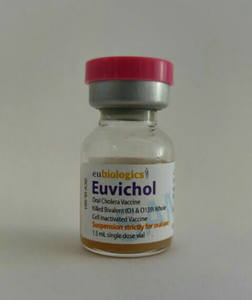
Last week, an oral cholera vaccine receivedWorld Health Organization (WHO) prequalification, a process of regulatory review which paves the way for procurement by United Nations agencies and international organizations. Up to 4.3 million cases and 142,000 deaths from cholera are reported annually, primarily in low- and middle-income countries, and the vaccine—Euvichol®—is 65 percent effective against the bacteria. Euvichol® was developed by GHTC member the International Vaccine Institute (IVI), and has been licensed to the Korean company Eubiologics, which plans to produce 6 million doses in 2016. The vaccine is a reformulated version of Shanchol™, which is licensed to Shantha Biotechnics in India, and was prequalified in 2011. According to the WHO, prequalification of Euvichol® could double the global supply of cholera vaccines.
Scientists have identified three compounds that could serve as the basis for a vaccine against onchocerciasis (river blindness). More than 17 million people globally are affected by river blindness, 70 percent of whom experience skin conditions, including itching and lesions, and 10 percent of whom develop eye conditions, leading to visual impairment. Without a vaccine, river blindness control programs use the antiparasitic drug ivermectin to treat the disease, however, ivermectin is not recommended for use in children under five. Additionally, in Central and Western Africa, where coinfection with Loa loa worms is common, use of ivermectin can lead to fatal brain inflammation. The team hopes to initiate safety and efficacy trials for a potential vaccine candidate by 2020 and 2025, respectively.
The Global Fund to Fight AIDS, Tuberculosis and Malaria is launching an “e-marketplace” through which global health product procurers (i.e., governments and nongovernmental organizations) can purchase everything from drugs and diagnostics to condoms and cars. The e-marketplace will be an online platform utilizing the Global Fund’s pooled procurement mechanisms. Through this mechanism, the Global Fund negotiates with manufacturers and purchases large quantities of global health commodities; governments and other organizations are then able to purchase products directly from the Global Fund, reducing product and transaction costs. This approach also improves demand forecasting and ensures a sustainable supply of products. The launch of the e-marketplace will be phased: while in the first quarter of 2016, only bednets will be available, artemisinin combination malaria medicines, malaria and HIV and AIDS diagnostics, and antiretrovirals will be available by the end of the year. The e-marketplace will initially be limited to Global Fund grant recipients, and 10 to 20 countries are expected to utilize the platform in 2016. The Global Fund hopes to open the e-marketplace to more buyers in the future and eventually offer products that are not part of Global Fund agreements, enabling procurers to independently compare quotes from different manufacturers.