Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
Global Health Now took an in-depth look at the flesh-eating fungus mycetoma—which enters the body through cuts, and can result in swollen, deformed limbs—in a three-part series on this Untold Global Health Story of 2015. The final installation focuses on GHTC member Drugs for Neglected Diseases initiative (DNDi), a product development partnership that will be initiating a clinical trial in mid-2016 to assess the safety and efficacy of the antifungal Fosravuconazole against mycetoma. Fosravuconazole is currently under development by Japanese pharmaceutical company Eisai, where it has proven more successful at combatting mycetoma in petri dishes than currently used medicines. The leading treatment, itraconazole, is only curative for one in thirteen patients after a year of use. The article notes that DNDi’s entire budget for the study is under US$2 million, compared with the average $1 billion spent by pharmaceutical companies. Eisai has agreed to donate Fosravuconazole pills for the trial which will compare Fosravuconazole, itraconazole, and a placebo.
Pharmaceutical company Gilead Sciences has been heavily criticized for “price gouging,” charging Americans $1,000 per pill for their drug sofosbuvir, which, for most patients, cures Hepatitis C in twelve weeks. However, in Egypt, where 10 percent of the population has chronic Hepatitis C—the highest rate worldwide—Gilead is selling them to the government for $10 per pill. The reduced price comes at a cost: to prevent the drugs from entering the black market and undermining Gilead’s sales, the Egyptian government has agreed to several restrictions that some advocates consider humiliating for patients and/or unethical. Not only are the drugs only sold at official government pharmacies, but patients must break the seal and take the first pill on-site to receive their first bottle and must return the original bottle to receive refills. The program has generated interest from other pharmaceutical companies, and the government has entered into similar deals with Bristol-Meyers Squibb and AbbVie, importing their medicines Viekira and Daklinza, respectively. Over the past year, more than 125,000 Egyptians have been treated, and the government hopes to treat another 300,000 each year starting in 2016, with the ultimate goal of reducing prevalence to 2 percent by 2025.
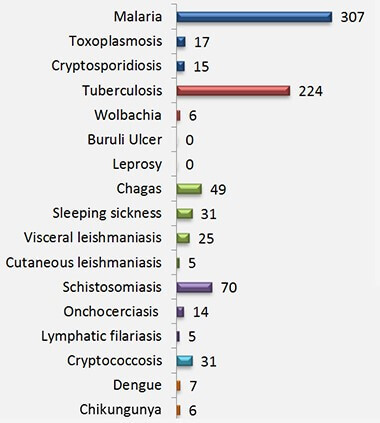
GHTC member Medicines for Malaria Venture (MMV) launched the Pathogen Box, a set of 400 compounds, each with proven activity (i.e., in test tubes) against neglected diseases, free for researchers and laboratories around the world. To create the Pathogen Box, MMV's team of chemists, as well as partners with expertise across neglected diseases, evaluated compounds in the European Bioinformatics Institute’s open access database, to determine those with the most potential. The team then acquired fresh samples of each compound to include in the boxes themselves. By expanding access to these samples, the Pathogen Box eliminates a major barrier to drug discovery for malaria, tuberculosis, schistosomiasis, Chagas, and other neglected diseases (See Figure 1).