In this guest post, the HIV Vaccines & Microbicides Resource Tracking Working Group—comprised of AVAC as secretariat, the
International AIDS Vaccine Initiative
, and UNAIDS—discusses its new report examining investment in research and development (R&D) for HIV prevention options.
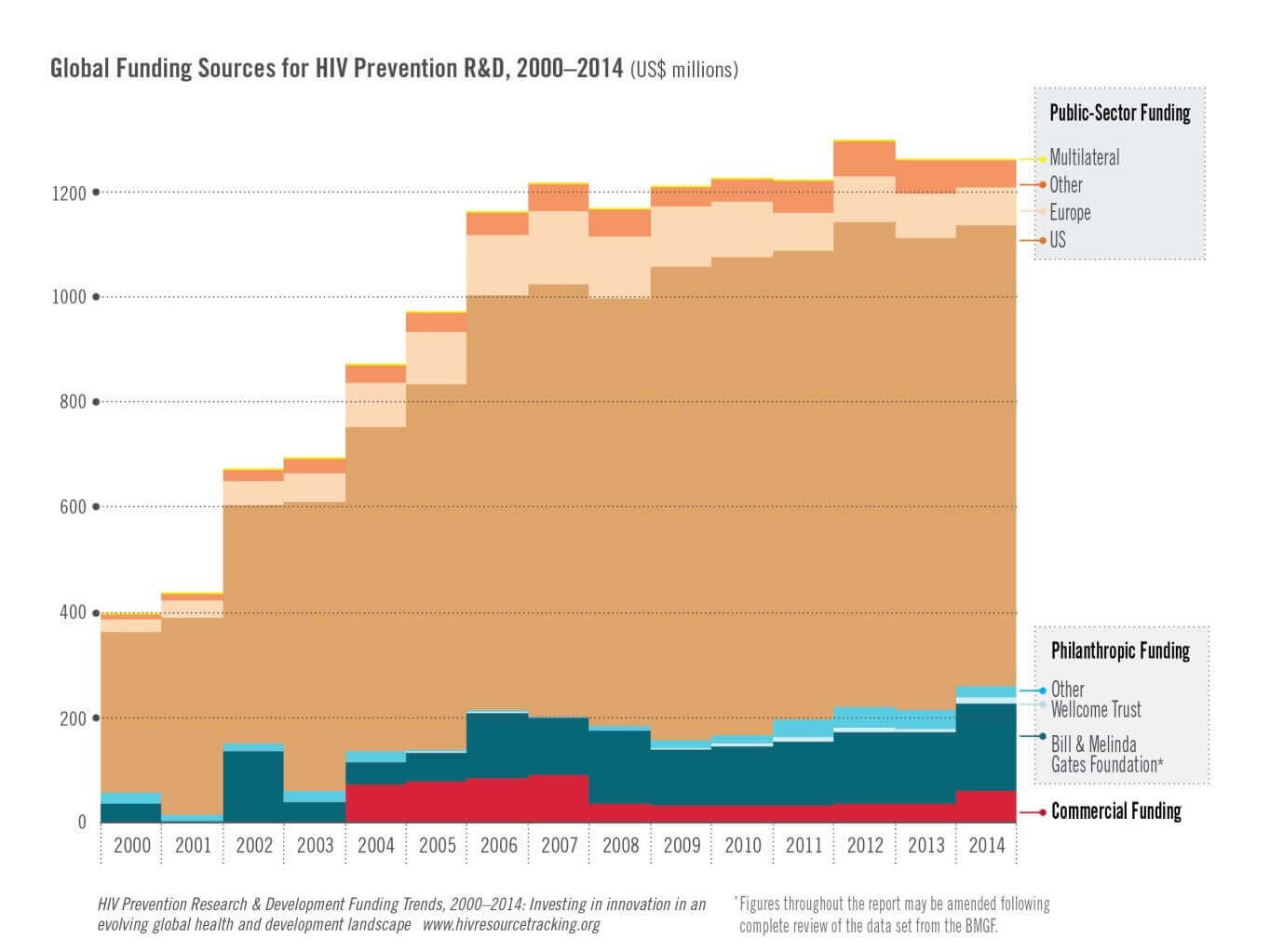
Investment in R&D for HIV prevention fell slightly, from US$1.26 billion to $1.25 billion, between 2013 and 2014 and has largely been stagnant
for nearly a decade, according to a new report from the HIV Vaccines & Microbicides Resource Tracking Working Group which looks at year-over-year
spending on HIV prevention R&D and trends spanning the last 15 years.
The report, HIV Prevention Research & Development Funding Trends 2000–2014: Investment Priorities to Fund Innovation in an Evolving Global Health and Development Landscape, provides detailed information on funding for R&D on HIV prevention options—including preventive HIV and AIDS vaccines, microbicides, pre-exposure
prophylaxis (PrEP), the use of AIDS treatment by people living with HIV to reduce transmission, the use of AIDS treatment to prevent infections
in infants born to women living with HIV, voluntary medical male circumcision, and female condoms. In addition to funding information for R&D
for HIV prevention options, the report also provides an update on investment in HIV cures, therapeutic vaccines, herpes (HSV-2) vaccines, and multipurpose
prevention technology research.
Despite the slight decline in funding, HIV prevention R&D is still delivering important advances. The 8th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention held in Vancouver from July 20-22, showcased results for a range of groundbreaking research that has been supported over the past several years,
including the Strategic Timing of Antiretroviral Treatment (START) trial, the
HPTN 052 antiretroviral (ARV) treatment as prevention trial, and several groundbreaking oral PrEP trials.
Results from studies of a vaginal ring containing the ARV dapivirine are expected in the next 12 months. Several different HIV and AIDS vaccine
candidates, neutralizing antibodies, and long-acting injectable ARVs are currently in studies that could lead to multiple efficacy trials starting
over the next two years. Robust ongoing investment will be critical to advancing these promising products through the R&D process.
Through their research and analysis, the Working Group identified four key findings about the state of funding for HIV prevention R&D which are
highlighted in the report:
- R&D investment is expanding beyond research to rollout: Since the Working Group began tracking investments in HIV prevention,
a number of options have moved through the pipeline from research to rollout. The importance of investing in products beyond bench science
and clinical trials is shown through the recent rollout and scale-up of options such as voluntary medical male circumcision and female condoms,
and—in the demonstration project phase—PrEP.
- Majority of investment is from several large funders: The United States remained the largest public-sector funder of HIV prevention
R&D, spending a total of $868 million in 2014—66 percent of the total investment. Combined, the US government and the Bill &
Melinda Gates Foundation accounted for 83 percent of all HIV prevention R&D funding. Expanding and diversifying this investment base could
bring in new perspectives and capacity and better assure sustained and consistent funding, reducing the potential negative impact of reductions
by one or two primary funders. Increasingly important to HIV prevention is research into how to deliver HIV prevention products and ensure
that those products meet the needs of, and reach, end users. Early investment to ensure the uptake of products is a growing part of HIV prevention
R&D.
- Decrease in number of philanthropic funders engaged: While the total amount of philanthropic funding increased in 2014, the number
of philanthropic funders engaged in HIV prevention research has been steadily declining since 2010. In 2014, 16 philanthropic funders invested
in HIV prevention research, down from 30 in 2010.
- Development funding priorities are changing: The Millennium Development Goals and the United Nations Declaration of Commitment on HIV and AIDS galvanized increased investment in HIV and AIDS. This in turn led to greater overall HIV prevention R&D investment—an increase of threefold
between 2001 and 2014. With the new Sustainable Development Goals set to be decided in September and global financing and development also
in a period of transition, it remains to be seen whether HIV prevention R&D, and global health R&D as a whole, will receive a prominent
place in the new international development agenda.
For further analysis of HIV prevention funding trends, visit the Working Group's website at www.hivresourcetracking.org to download HIV Prevention Research & Development Funding Trends 2000–2014: Investing in innovation in an evolving global health and development landscape and to view additional Working Group reports,
as well as graphics and
PowerPoint slides.

