Claire WingfieldPATH
Claire Wingfield is a Product Development Policy Officer within the Advocacy and Public Policy Department at PATH, a global nonprofit dedicated to ending health inequity.
In this guest post, Claire Wingfield—product development policy officer at PATH—writes about a new paper she developed for the GHTC that outlines key issues surrounding access to global health products.
In order to achieve impact, a health technology must be more than just safe and effective. It must also be affordable, available, and acceptable to ensure that it is accessible to those who need it most.
The pipeline of products addressing public health needs in low- and middle-income countries (LMICs) has grown substantially over the past two decades. Much of that success can be traced back to the 1990s when a number of nonprofit product development organizations (NPPDs) were established to accelerate the development and accessibility of new health technologies targeting poverty-related diseases and conditions. Since that time, NPPDs and their partners have contributed to the development, evaluation, and/or introduction of 42 new health products.
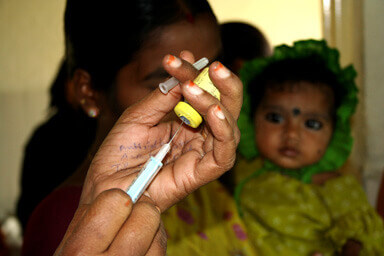
Because NPPDs were established to develop—not deliver—health technologies, they negotiate collaborations with partners from the public and private sectors that are designed to ensure access to the resulting products and knowledge. Today, the GHTC is launching a paper that explores how NPPDs approach access and how they manage partnerships and negotiate agreements to improve access – which they define as the affordability, availability, and acceptability of technologies targeting the health needs of LMICs.
Although there is no “one size fits all” model for how NPPDs operate, they do employ some common strategies to ensure that the resulting products benefit those most in need:
NPPDs also shared lessons that they and their partners have learned in trying to improve the affordability, availability, and acceptability of new health technologies for those most in need:
Any delays in planning and implementing an access strategy can result in significant lag time from licensure to actual delivery in country. A product that needs to be retrofitted for affordability, availability, and acceptability will result in a costly and time-consuming development process. Therefore, as the GHTC’s new paper reveals, it is critical that NPPDs and partners address these principles from the beginning of the product development process.