Earlier this week, the Global Health Technologies Coalition (GHTC) and partners brought more than 25 innovative global health technologies to Capitol Hill to highlight how US government investments in global health research and development (R&D) are saving lives in the United States and around the world. Each technology displayed—whether a vaccine, diagnostic, drug, device, or nutrition solution— demonstrate how these investments not only protect the world’s most vulnerable people but also strengthen America’s own health security, help prevent pandemics, and fuel high-quality US jobs and economic growth.
The exhibit offered a snapshot of a larger story of how the United States is doing well by doing good. Between 2007 and 2022, the US government invested just under $46 billion in global health R&D. These investments helped drive the development of 67 new technologies approved since 1999 for neglected and (non-COVID-19) emerging diseases. At the same time, they created 600,000 new US jobs and yielded knowledge expected to generate more than $255 billion in long-term benefits for the American economy—a six-fold return on investment.
This exhibit showcased what’s possible when science, purpose, and smart policy align. Explore the exciting US-backed breakthroughs displayed below.

VACCINES
Single-dose vaccines for Sudan and Marburg viruses on the rVSV vaccine platform
|
 | IAVI, a nonprofit based in New York and New Jersey, is advancing single-dose vaccines for the Sudan and Marburg viruses using the proven rVSV vaccine platform, the same technology behind an Ebola vaccine that has helped contain recent outbreaks. Sudan and Marburg viruses continue to spark deadly, Ebola-like outbreaks, yet no approved vaccines or treatments exist, underscoring the urgency of this work. These vaccine candidates are being developed with critical support from the Defense Threat Reduction Agency at the US Department of Defense (DoD) and the Biomedical Advanced Research and Development Authority (BARDA).
|
Novel oral polio type 2 (nOPV2) vaccine
|
 | PATH, a nonprofit developer based in Washington state with an office in the District of Columbia, alongside the University of California, San Francisco; the Washington-based Gates Foundation; and global partners, developed nOPV2, a next-generation oral polio vaccine. nOPV2 maintains the same advantages of the original oral vaccine but has been specifically engineered to be less likely to seed vaccine-derived polio outbreaks in communities with low immunization coverage. This breakthrough, which is accelerating global efforts to end polio, also benefitted from critical development support from the US Centers for Disease Control and Prevention (CDC).
|
Next-generation malaria vaccines
|
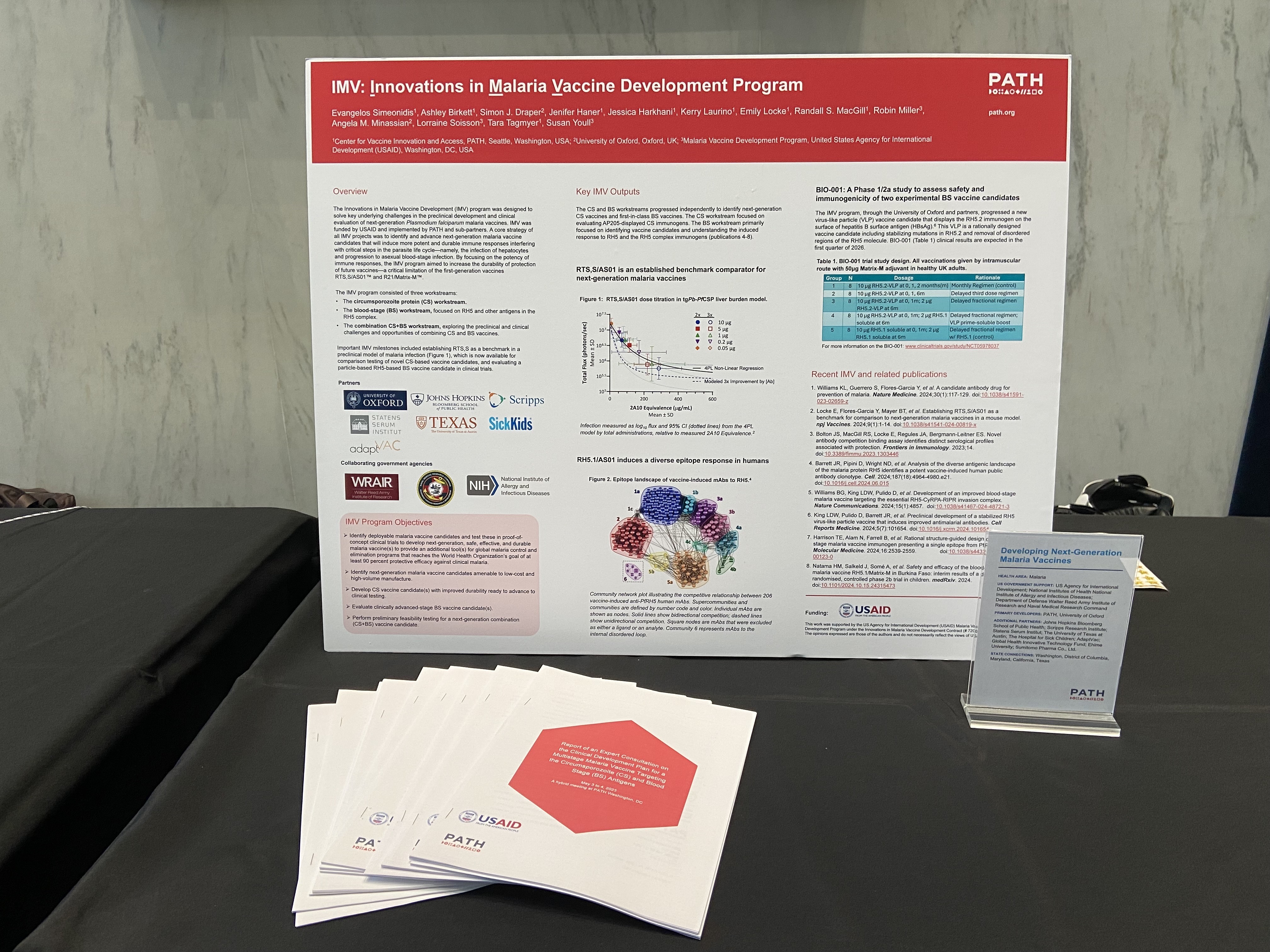 | PATH and the University of Oxford are developing next-generation malaria vaccines designed to overcome key limitations of the currently available options and help turn the tide of this longstanding global health challenge. This work has received vital US government support from the US Agency for International Development (USAID), the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH), and the DoD Walter Reed Army Institute of Research and Naval Medical Research Command. Additional US-based partners, including Johns Hopkins University in Maryland, the Scripps Research Institute in California, and the University of Texas at Austin, alongside a constellation of international collaborators, also played a critical role in advancing this research.
|
DRUGS
Pretomanid, a novel tuberculosis (TB) drug
|
 | TB Alliance, a New York-based nonprofit, developed pretomanid, a groundbreaking drug for treating highly drug-resistant TB. As part of a six-month combination regimen, it has drastically reduced treatment duration and complexity for patients compared to previous regimens that required up to 20 or more pills a day—as many as 14,000 total over the course of 18 months or more of treatment. This work was backed by US federal funding from USAID and NIAID and advanced in partnership with a broad coalition of American companies, nonprofits, and universities, including AbbVie Inc. in Illinois, Advancing Clinical Therapeutics Globally for HIV and Other Infections in the District of Columbia, the Gates Foundation, Harvard University in Massachusetts, Johns Hopkins University, Johnson & Johnson in New Jersey, RTI International in North Carolina, Texas A&M University, the University of Illinois Chicago, and the University of North Carolina at Chapel Hill.
|
Child-friendly TB medicines
|
 | TB continues to infect millions of people worldwide each year, with children facing a heavy burden—an estimated 1.3 million new pediatric cases were reported in 2023. For decades, children with TB were treated with crushed adult pills, a practice that was difficult for caregivers and often led to inconsistent dosing. TB Alliance developed child-friendly TB medicines that are palatable, fast-dissolving in water, and precisely dosed for children, ensuring young patients finally have access to safe, appropriate, and easy-to-administer therapies. This work received vital funding from USAID and was advanced in partnership with other international donors and organizations, as well as US-based partners, including Management Sciences for Health in Virginia and Baylor College of Medicine in Texas.
|
| Inhaled oxytocin for the prevention and treatment of postpartum hemorrhage |
 | Postpartum hemorrhage, or excessive bleeding after childbirth, is the leading cause of maternal deaths globally. To address this urgent challenge, Monash University has developed an investigational heat-stable, inhaled powder formulation of oxytocin designed to provide the same protection as the injectable gold standard treatment. By eliminating the need for refrigeration and trained providers to administer injections, this innovation could greatly expand access to lifesaving care for mothers in low-resource settings. This work received funding from USAID and support from US-based partners, including the Gates Foundation and Johnson & Johnson, alongside other international collaborators.
|
| Monoclonal antibody therapy to prevent and treat Nipah virus (MBP1F5) |
 | The Coalition for Epidemic Preparedness Innovations, whose US headquarters are in the District of Columbia, is supporting the development of a monoclonal antibody therapy being advanced by California-based Servare GMP and Mapp Biopharmaceutical to prevent and treat Nipah virus, a highly lethal zoonotic disease with no approved treatments or vaccines. This work received support from the DoD Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense and Uniformed Services University, as well as USAID.
|
| Tafenoquine, a single-dose therapy for relapse prevention of P. vivax malaria & long-acting injectable malaria prophylaxis |
 | The Medicines for Malaria Venture and GSK—whose US headquarters are in Pennsylvania— developed tafenoquine, a single-dose treatment to prevent the difficult-to-treat relapses characteristic of Plasmodium vivax malaria. By replacing multi-day regimens, tafenoquine simplifies care for patients and helps malaria-endemic countries accelerate progress toward elimination. Its development received crucial support from USAID, the DoD Walter Reed Army Institute of Research, and the US Food and Drug Administration through its Priority Review Voucher Program.
The Medicines for Malaria Venture, Oregon Health & Science University, and the California-based Calibr-Skaggs Institute for Innovative Medicines are co-developing a long-acting injectable malaria prophylaxis designed to provide combination protection against both Plasmodium falciparum and blood-stage P. vivax malaria. This next-generation approach aims to offer durable, simplified prevention for people in high-risk areas. The work received important support from USAID and the Department of Veterans Affairs. |
| Injectable lenacapavir for HIV prevention |
 | Gilead Sciences, a California-based biopharmaceutical company, developed lenacapavir, a groundbreaking new HIV prevention drug requiring twice-yearly injections. In pivotal Phase 3 trials, lenacapavir demonstrated remarkable efficacy across key populations, including historically underserved groups. Developed using evidence from NIH-funded research, it offers discreet, long-acting protection, providing an alternative to daily pills or bimonthly injections and making HIV prevention more accessible and easier to sustain.
|
DEVICES
Ellavi Uterine Balloon Tamponade for postpartum hemorrhage
|
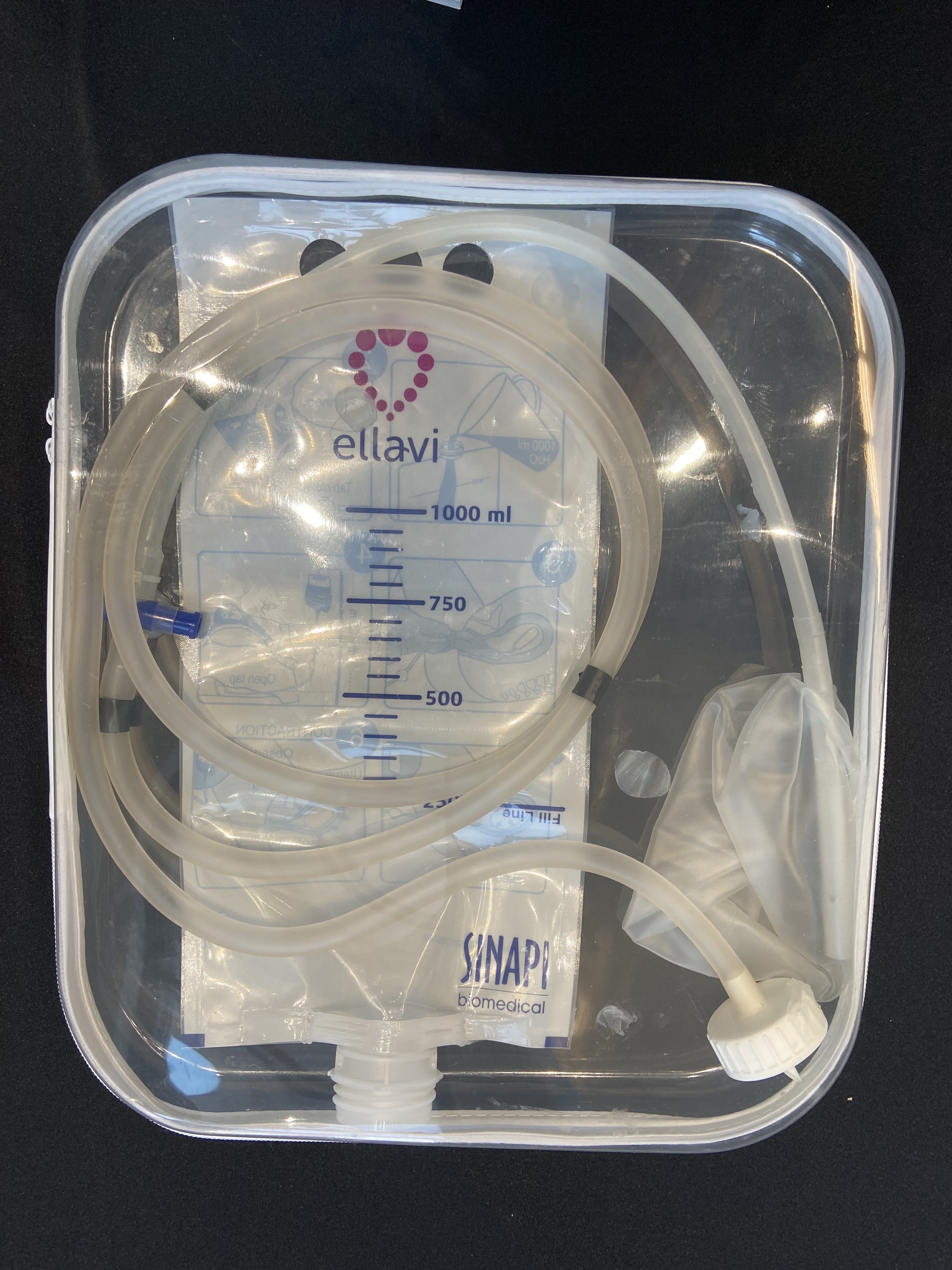 | As noted earlier, postpartum hemorrhage is the leading cause of maternal deaths worldwide. The Ellavi Uterine Balloon Tamponade is a low-cost, easy-to-use inflatable device placed inside the uterus that uses pressure to rapidly slow or stop bleeding. Designed specifically for use in low-resource settings, it offers a simple, lifesaving intervention where advanced care is not always available. The device was developed by PATH and Sinapi Biomedical, with support from USAID, the Gates Foundation, and other partners.
|
Anne® One continuous wireless vital sign monitoring platform
|
 | Life-threatening pregnancy risks can too often go undetected in areas with limited resources and health care workforce. The Anne® One continuous, wireless vital-sign monitoring platform, developed by Illinois-based Sibel Health, Inc. and supported by the Gates Foundation and the Steele Foundation for Hope in New Hampshire, helps identify complications in real time, helping improve care for both mothers and babies. The technology can also enhance monitoring and care for patients of all ages, whether at home or in hospital settings. The work received key US government support from the Small Business Innovation Research program and the NIH RADx for Maternal Health Challenge, BARDA, and the DoD Medical Technology Enterprise Consortium.
|
| Monthly dapivirine vaginal ring for HIV prevention |
 | The dapivirine vaginal ring, originally developed by the Maryland-based International Partnership for Microbicides and now advanced by the Population Council in New York, is a flexible silicone ring that users self-insert, which reduces the risk of HIV infection. The ring provides one month of protection by slowly releasing the antiretroviral drug dapivirine, offering a discreet, user-controlled prevention option. Its development received critical support from NIH, USAID, the Gates Foundation, and other international donors and partners.
|
| Microarray needle-free “button” for vaccine and drug delivery |
 | Micron Biomedical, based in Georgia, developed a microarray needle-free “button” that could one day be used for delivering vaccines and pharmaceuticals without the need for traditional injections. This innovative, pain-free solution could be self-administered, reducing barriers to vaccination and treatment. It also limits or eliminates the need for refrigerated storage, simplifies transport and storage, and produces no sharps waste, making it especially valuable for low-resource settings. This technology received US funding support from CDC and BARDA (through the New York-based Global Health Investment Corporation) and was developed in partnership with other US-based groups, including the Gates Foundation, PATH, and Emory University in Georgia.
|
Nifty Cup
|
 | Millions of babies are born each year who are unable to breastfeed because they were born prematurely or have conditions such as cleft lip or palate. In low-resource settings, commonly used feeding tools can be impractical or difficult to keep hygienic. To address this challenge, PATH partnered with Washington-based collaborators, The University of Washington School of Dentistry and Seattle Children’s Hospital, with support from USAID and Laerdal Global Health, to develop the Nifty Cup. This low-cost silicone feeding cup is durable, gentle, easy to disinfect and simple for caregivers to use—a great example of a frugal, high-impact innovation designed specifically for low-resource environments.
|
HEATmarker™ vaccine vial monitor
|
 | Most vaccines require continuous refrigeration to remain effective, which can be a significant challenge in remote and low-resource settings worldwide. Breaks in the cold chain can lead health workers to discard perfectly good vaccines because they suspect heat damage or, worse, unknowingly administer doses that have lost potency. To solve this, PATH and New Jersey-based Temptime (later acquired by Illinois-based Zebra Technologies) developed the vaccine vial monitor, a small sticker placed on vaccine vials that changes color when exposed to too much heat, clearly indicating whether a vaccine is still safe to use, preventing waste and saving lives. This simple, low-cost innovation reduces waste, improves safety, and helps ensure lifesaving vaccines reach the people who need them. The work received crucial support from USAID.
|
BD Uniject™ auto-disable prefillable injection system
|
 | Injectable drugs and vaccines have saved countless lives, yet administering them safely in low-resource settings presents challenges—health workers require appropriate training and if reused, syringes can spread dangerous infections. With funding from USAID, PATH designed the Uniject™ system, now manufactured by New Jersey-based BD. It’s an inexpensive, pre-measured and -filled syringe that automatically disables after a single use. This technology enables health workers with minimal training to safely deliver correctly dosed injections, prevents syringe reuse, reduces product waste, and ultimately saves lives and costs. |
BD SoloShot™ auto-disable syringe
|
 | BD and PATH also partnered on the SoloShot™ auto-disable syringe, with support from USAID, which locks after injection, also helping prevent reuse and bloodborne disease transmission.
|
| Interceptor® G2 dual-insecticide bednet |
 | As rising insecticide resistance threatens progress against mosquito-borne diseases such as malaria, Zika, and dengue, the Interceptor® G2 dual-insecticide treated bednet represents an important evolution of one of global health’s most essential prevention tools. Developed by BASF, whose agriculture division is based in North Carolina, the Interceptor® G2 uses two insecticides to improve effectiveness against resistant mosquito populations. Its development was supported by the President’s Malaria Initiative, USAID, and CDC, as well as the Gates Foundation, the Innovative Vector Control Consortium, and other international partners.
|
DIAGNOSTICS
Lumify AI-assisted handheld ultrasound
|
 | Life-threatening risks during pregnancy can go undetected in areas with limited health care resources, leading to deaths, as well as complications for both mothers and their babies. But a portable, artificial-intelligence-powered ultrasound developed by Philips Healthcare, whose North America offices are located in Massachusetts, with support from BARDA and the Gates Foundation, is addressing this challenge head on by helping improve access to screening for pregnant mothers in low-resource settings.
|
| Determine™ Antenatal Care Panel; Determine™ HIV Early Detect, Determine™ HBsAg 2, and Determine™ Syphilis TP rapid tests |
 | Illinois-based company Abbott developed a rapid panel test capable of simultaneously detecting HIV, hepatitis B virus, and syphilis, three major infections that pose serious risks to maternal and newborn health. The combination platform, which could simplify testing for health systems and patients and improve testing coverage, builds on individual in vitro rapid tests for each virus, which were developed by Abbott and later procured and scaled with support from the US President’s Emergency Plan for AIDS Relief (PEPFAR).
|
OraQuick™ Ebola Rapid Antigen Test, Investigational Marburg rapid antigen test, and OraQuick™ HIV Self-Test
|
 | OraSure Technologies, a Pennsylvania-based diagnostics company developed the OraQuick™ Ebola Rapid Antigen Test, a point-of-care tool that enables fast, presumptive diagnosis of Ebola virus disease. By providing results in minutes, the test supports health workers in rapidly isolating patients, initiating potentially lifesaving treatment, and ensuring safe and dignified burials that reduce transmission risk. The test was developed with support from BARDA.
With support from BARDA, OraSure is also advancing a similar rapid antigen test for Marburg, a related virus to Ebola that also causes sever and often fatal disease.
OraSure also has an oral HIV self-test, built on the same platform as its Ebola and Marburg tests. As the first US Food and Drug Administration-approved HIV self-test, it provides discreet, rapid testing for patients at home in as little as 20 minutes. The US government has supported scale-up of this product globally through PEPFAR. |
| Co-Dx PCR Pro™ for on-site PCR testing |
 | The PCR Pro™ molecular diagnostic platform from Utah-based Co-Diagnostics is designed to enable health care professionals to conduct on-site PCR testing, expanding access to fast, accurate testing outside traditional laboratory settings. With test panels available for flu, COVID-19, and RSV as a multiplex test and, separately, HPV and TB, the platform—developed with support from the NIH RADx program, as well as from PATH and the Gates Foundation for specific applications—has the potential to transform decentralized testing globally.
|
NUTRITION INNOVATIONS
| Ready-to-use therapeutic food for the treatment of severe acute malnutrition |
 | Nearly one third of children under five worldwide suffer from malnutrition. But a ready-to-use therapeutic food Plumpy’Nut has been a game changer. Developed by scientists at Washington University in St Louis and the French Institut de Recherche pour le Développement, manufactured by Georgia-based Mana and New Hampshire-based Edesia Nutrition, and scaled up with support from USAID and now the Department of State, this essential mix of vitamins, milk, and peanut butter allows severely malnourished children to recover and stay recovered with just a few servings.
|
| Vitamin A biofortified corn |
 | Vitamin A deficiency is the leading cause of preventable child blindness worldwide. It impairs growth and immunity, increasing the risk of severe illness from common childhood infections such as diarrhea and measles. Corn biofortified with Vitamin A, developed by the District of Columbia-based Harvest Plus program at the International Food Policy Research institute, with support from USAID’s Feed the Future program, has been a transformative innovation. When consumed regularly, it can provide up to half of daily vitamin A needs for women of reproductive age and children. |
Thanks to our partners the American Society for Microbiology, the American Society of Tropical Medicine and Hygiene, Research!America, the Innovative Vector Control Consortium, IAVI, Monash University, Philips, Co-Diagnostics, HarvestPlus, Micron Biomedical, BASF, the Medicines for Malaria Venture, TB Alliance, Abbott, Mana, BD, Zebra Technologies, Sibel Health, the Coalition for Epidemic Preparedness Innovations, PATH, Gilead Sciences, OraSure Technologies, and the Global Health Investment Corporation for supporting this event.

























