Matthew RobinsonGHTC
Matthew Robinson is a policy and advocacy officer at GHTC who leads the coalition's multilateral advocacy work.
 Researchers examine onchocerciasis (river blindness) diagnostic tests. PATH/Patrick McKernWithout the right diagnostics, we can’t effectively treat diseases. It’s an obvious statement. But in many low- and middle-income countries, doctors and
health care workers don’t have access to the diagnostic tools they need, often because existing tests are too costly and not appropriate for low-resource
settings or tests don’t exist at all. Without the right tests, doctors end up relying on patient symptoms, which can be remarkably similar across different
diseases. The result can be devastating: A wrong diagnosis can lead to the wrong treatment or a failure to recognize the presence of disease with pandemic
potential. This problem is a common one: in half of African countries, more than 80 percent of those treated for malaria receive medicine without confirmation
of a diagnostics, and during the 2014–2015 Ebola outbreak, efforts to contain the outbreak were hampered by a lack of rapid, point-of-care tests that
could be deployed easily in the field.
Researchers examine onchocerciasis (river blindness) diagnostic tests. PATH/Patrick McKernWithout the right diagnostics, we can’t effectively treat diseases. It’s an obvious statement. But in many low- and middle-income countries, doctors and
health care workers don’t have access to the diagnostic tools they need, often because existing tests are too costly and not appropriate for low-resource
settings or tests don’t exist at all. Without the right tests, doctors end up relying on patient symptoms, which can be remarkably similar across different
diseases. The result can be devastating: A wrong diagnosis can lead to the wrong treatment or a failure to recognize the presence of disease with pandemic
potential. This problem is a common one: in half of African countries, more than 80 percent of those treated for malaria receive medicine without confirmation
of a diagnostics, and during the 2014–2015 Ebola outbreak, efforts to contain the outbreak were hampered by a lack of rapid, point-of-care tests that
could be deployed easily in the field.
In a recent New England Journal of Medicine article, a group of scientists and doctors suggested an innovative idea to improve global access to critically-needed diagnostic tools: create a list.
More specifically, they’re calling for the creation of an essential diagnostics list to complement the World Health Organization’s (WHO) Model List of Essential Medicines (EML). The EML provides guidance to countries on what drugs, therapies, and treatments should be made available to citizens to meet basic public health needs. Likewise, an essential diagnostics list would provide guidance on the vital diagnostics tests that should be available in each country, depending on its disease burden. In this way, the two lists would support and complement each other, with the diagnostics list identifying the tests needed to enable appropriate use of medicines on the EML.
The authors of the article have detailed the many ways an essential diagnostics list could be a transformative force for improving diagnostic capacity and access. By setting global guidance on the diagnostics tests recommended to countries to address basic health needs, an essential diagnostics list could provide a forceful policy argument for governments to invest in making existing diagnostics more broadly available. A list could also encourage governments to make further improvements to health systems to support diagnostic use, including laboratory training programs and strengthening health infrastructure and supply chains.
But what GHTC is most excited about is one of the lesser-discussed aspects of an essential diagnostics list: its potential to catalyze research and development (R&D) of new diagnostic tools.
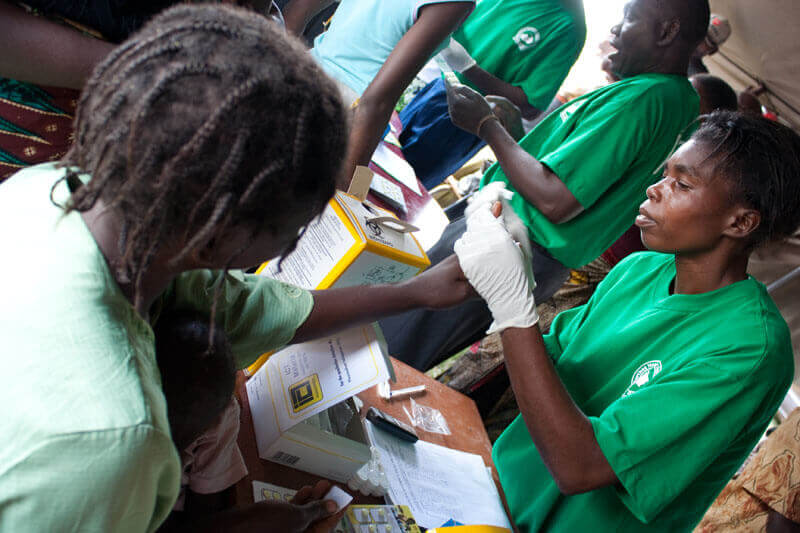 A patient gets tested for malaria. PATH/Gena Morgan
A patient gets tested for malaria. PATH/Gena Morgan
Diagnostic R&D is a significantly underfunded area of research, accounting for less than 5 percent of global funding of neglected disease R&D. The result is a lack of diagnostic tools targeting the specific health needs of low- and middle-income countries, such as easy-to-use platform technologies or rapid, point-of-care tests that can be used in remote, rural clinics.
By detailing which diagnostics are necessary to support health interventions on the EML, an essential diagnostics list would readily expose health areas where we today lack appropriate and effective tools. In this way, the list could signal to product developers, industry, and donors where funding and attention is needed to facilitate diagnostic R&D.
The impact of improved diagnostic access and capacity would be considerable: better care and treatment for patients, reduction in the inappropriate use of medicines which is contributing to drug resistance, and improved ability to detect emerging diseases like Zika and Ebola early before they become epidemics.
On Wednesday, GHTC sent a letter, signed by 11 of our member organizations, to WHO’s assistant director-general for Health Systems and Innovation calling for WHO to spearhead the creation of an essential diagnostics list. Given WHO’s experience in administering the EML and its ongoing work in providing guidance on diagnostic testing and laboratory accreditation; we believe the organization is uniquely positioned to lead on the development and implementation of this list. But making this initiative a reality will require the support of civil society and member states, as well as additional funding to stand-up the program at WHO.
It is clear that treatment can be improved with essential diagnostics—ones we have today and others yet to be developed. We urge WHO and its member states to heed this call to establish an essential diagnostics list. It’s a smart, cost-effective idea that could advance diagnostic R&D and access.
Update: On November 18, Dr. Marie-Paule Kieny, WHO Assistant Director-General for Health Systems and Innovation, replied to GHTC's letter indicating that WHO is pursuing analysis of the idea and outlining next steps. GHTC will continue to monitor progress and engage with WHO in support of this initiative.