Drug Development ProgramPATH
The Drug Development Program at PATH works to advance the most promising medicines for a range of urgent global health challenges.
Drug development is a long and complex process. Estimates of the cost to develop a drug can range from $150 million to $1.3 billion up to the point of regulatory approval, and the process can take upward of a decade.
In this guest post, PATH’s drug development program provides a step-by-step overview of what goes into each phase of drug development. The program’s investigational new drug for diarrheal disease, iOWH032 (currently in phase 2 trials), serves as an example. This is the first post in a three-part series.
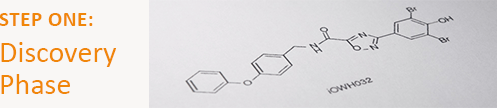
Break down a disease and find a promising agent
At this stage, researchers examine the mechanisms by which a disease affects normal physiological processes, in order to determine which mechanism provides the most attractive target for treatment.
In consultation with an international advisory board of clinicians, scientists, and public health experts, our team selected the CFTR chloride channel as the best target to combat the deadly dehydration caused by diarrhea. From 2007 to 2010, we partnered with private pharmaceutical companies Roche, Novartis, and BioFocus DPI to screen nearly 2 million molecules from their chemical libraries. Forty drug leads were identified for further research, and one, iOWH032, was selected for further development.
 Assess safety and biological activity in laboratory and animal studies
Assess safety and biological activity in laboratory and animal studies
Before a drug can be tested with healthy volunteers, scientists must first assess the safety and biological activity of the drug in a laboratory setting, according to US Food and Drug Administration (FDA) standards.
From 2010 to 2011, our team conducted toxicology studies in several animal species and found iOWH032 to be safe, even at very high doses. Additionally, iOWH032 reduced cholera toxin-induced intestinal secretion in several animal models and in vitro.
 Submit your chemical and manufacturing data, preclinical test results, and the rationale and strategies for clinical studies
Submit your chemical and manufacturing data, preclinical test results, and the rationale and strategies for clinical studies
After preclinical testing is completed, scientists are able to submit chemical and manufacturing data and preclinical test results, as well as the rationale and strategies for clinical studies, to the FDA in an Investigational New Drug (IND) application.
On December 27, 2010, we submitted an IND for iOWH032, the first submission of an IND for a completely new chemical entity by a small nonprofit organization. We received rapid clearance by the FDA on February 4, 2011.
 Determine a compound’s safety and pharmacology in healthy volunteers
Determine a compound’s safety and pharmacology in healthy volunteers
With necessary ethical reviews completed and the IND granted by the FDA, drug developers can proceed to clinical trials in healthy volunteers. At this stage, clinician-scientists can begin to determine a compound’s safety and clinical pharmacology.
Between 2011 and 2013, we partnered with Quintiles, a clinical research organization based in Kansas, to conduct two studies evaluating iOWH032’s safety and tolerability, as well as measuring the extent and rate of its absorption, distribution, metabolism, and excretion. The studies involved 92 healthy volunteers and concluded that iOWH032 was safe and well tolerated. No serious adverse events were reported.
During 2013, we conducted a study in partnership with the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) to assess the pharmacokinetics, safety, and tolerability of iOWH032 in eight healthy Bangladeshi volunteers. Reports of the drug’s safety and tolerability, as well as the pharmacokinetic profile, were very similar to the results concluded from the studies of healthy American volunteers, and no serious adverse events were reported.
 Evaluate a compound’s effectiveness and side effects in cholera patients
Evaluate a compound’s effectiveness and side effects in cholera patients
In phase 2 trials, a drug is evaluated for its effectiveness and potential side effects in patient volunteers.
Beginning this year and continuing into 2014, iOWH032 is in phase 2 clinical trials. Ongoing studies are being conducted at icddr,b to assess the pharmacokinetics, safety, and efficacy of iOWH032 in patients with severe cholera.
 Verify a compound’s effectiveness and safety and confirm dosage in patient volunteers
Verify a compound’s effectiveness and safety and confirm dosage in patient volunteers
At this phase, researchers seek to verify a compound’s effectiveness and safety, in addition to confirming dose and schedule in patient volunteers. During this final step before FDA approval, researchers confirm previous findings in a larger population. Phase 3 can last from 2 to 10 years, and involve hundreds or thousands of patients across multiple sites.
 Submit a new drug application to the FDA
Submit a new drug application to the FDA
Once phase 3 is completed, a New Drug Application (NDA) can be submitted to the FDA. Information on the chemical makeup and manufacturing process, pharmacology and toxicity of the compound, human pharmacokinetics, results of the clinical trials, and proposed labeling is provided. Once the NDA is approved, the drug can be marketed.
 Conduct post-marketing surveillance
Conduct post-marketing surveillance
In phase 4 trials, drug developers conduct post-marketing surveillance, without major enrollment restrictions, to examine the risks and benefits of a new drug in a larger patient population. Phase 4 studies may be conducted to evaluate the long-term effects of a drug over an extended period of time in a larger group or to continuously discover more information about the drug, including benefits in other areas (beyond what is on the approved label).
Photos: 1, 3, and 7, PATH Drug Solutions; 2 and 4, PATH/Patrick McKern; 5, 6, and 8, ©Jonathan Torgovnik.